Leaf Anatomy in Relation to C3 and C4 Pathways of CO2 Fixation
Introduction
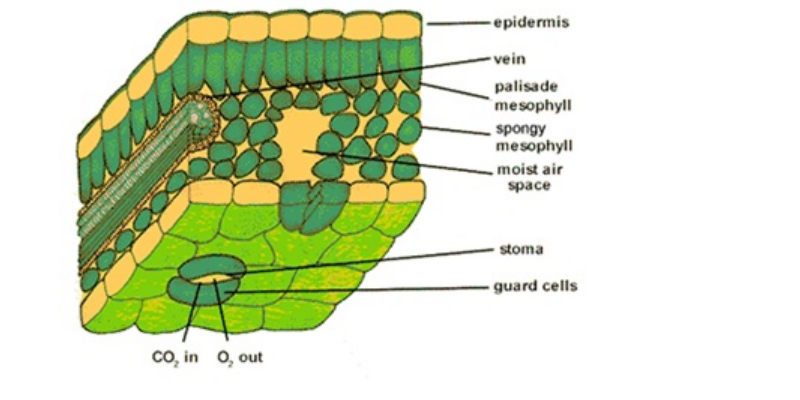
Leaf anatomy may influence net leaf photosynthesis to a large degree and thus cause great differences in light-use efficiency. Like the root and stem, the leaf consists of vascular, parenchymatous and dermal tissue, the latter being a persistent epidermis. …
Leaf Anatomy in Relation to C3 and C4 Pathways of CO2 Fixation Read More »