Synthesis of Tetraamminecopper(iI) Sulphate Monohydrate
Introduction
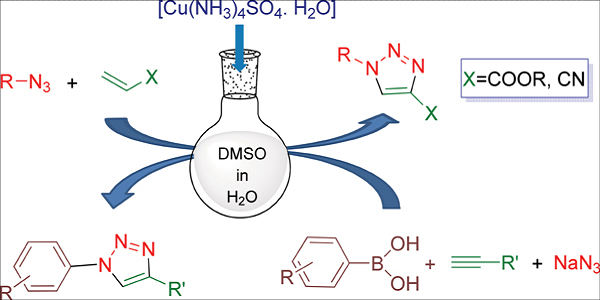
Tetraaminecopper(II) sulphate monohydrate is an inorganic complex compound with the formula [Cu(NH3)4]SO4.H2O. In this experiment,tetraaminecopper(II) sulphate monohydrate is prepared from a reaction of CuSO4.5H2O and NH3…
Synthesis of Tetraamminecopper(iI) Sulphate Monohydrate Read More »