Introduction
Thermochemistry deals with the experimental measurement and calculation of heat changes. Every chemical reaction is always accompanied by a change in energy usually in form of heat: a reaction will either give off heat (exothermic), or it will absorb heat (endothermic). Many times the heat transfer associated with a chemical reaction is more important than the reaction itself. The heat change that accompanies the reaction of one mole of substance is designated by the symbol Q. Heat changes for chemical reactions are usually given in calories or joules. A calorie is the quantity of heat required to raise the temperature of one gram of water one degree centigrade and one calorie equals 4.18 joules.
Theory
The heat of neutralization (DHN) is the change in enthalpy that occurs when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to form water and a salt. It is a special case of the heat of reaction. It is defined as the energy released with the formation of 1 mole of water.
The basic reaction in a neutralization process is;
H+ + OH– → H2O + ∆HN
The acid produces the H+ via the equilibrium process;
HnA → nH+ + An-
where n = 1, 2, 3 …
The quantity of H+ produced by an acid in solution depends on the strength of the degree of dissociation of the acid in the particular solution.Some acids dissociate completely in aqueous solution and readily produce hydrogen ions and are called strong acids, while weak acids are partially dissociated in aqueous solution. The dissociation reaction is an equilibrium process and energy is required to produce hydrogen ions. For weak acids or bases, the heat of neutralization is pH dependent.
HCl +H2O ↔ H3O+ + Cl– strong acid dissociation
CH3COOH +H2O ↔CH3COO– + H3O weak acid dissociation
The heat of neutralization can be calculated using the equation:
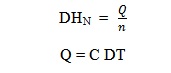
where:
n = no of moles of the limiting reagent between H+ and OH–
Q = heat released during reaction
C = heat capacity of the calorimeter (constant)
DT = change in temperature
DT = Tf – Ti
where:
Ti = initial temperature
Tf = final temperature
Equipments and Materials Needed
Apparatus
- Burette
- Pipette
- Calorimeter
- Stirrer
- Thermometer
- Glass Tube
- Retort Stand
Reagents
- A known concentration of acid (e.g 3.0 M HCl)
- A known concentration of base (e.g 2.0 M NaOH)
Procedures
- Clamp the burette on the retort stand and fill with acid.
- Measure a known volume of the base (e.g 30 mL) into the reaction vessel of the calorimeter and fix in the glass tube well above the base. Allow both solutionsto sit for 5 minutes, then measure the temperature of each. Average these two temperatures and record this value as Ti [Always record temperatures to the nearest 0.1°C.].
- Quickly add the same volume of acid through the glass tube to the base in the reaction vessel. Monitor the temperature of the calorimeter and record the highest temperature it reaches. This will be your value of Tf.
- Repeat the procedure if using multiple acids of different strengths.
Results and Calculations
Use the above equations to calculate the heat of neutralization using the obtained initial and final temperature as Ti and Tf respectively.
Discussion and Conclusion
A chemical reaction is often accompanied by a heat change. Neutralization involves the combination of H+ and OH– ions to form unionized water. The heat of neutralization of strong acids are considerably higher than the heat of neutralization of weak acids.
References
- Experimental Physical Chemistry Seventh Edition MC Grawhill book Company
- Slowinski, E.J., Wolsey, W.C. andMasterton, W.L. (2001),“Chemical Principles in theLaboratory”, 7th ed.Harcourt: Fort Worth.
- “Enthalpy of Neutralization”. www.wikipedia.com. January 8, 2018.
- Neidig, H.A. and Spencer, J.N. (1989),“Heat of Neutralization. In Modular Laboratory Programing Chemistry”,Neidig, H.A., Ed. Chemical Education Resources, Inc.: Palmyra, PA.
Download “Procedure - Determination of Heat of Neutralization”
Download “Report - Determination of Heat of Neutralization”