Introduction
Hydrates are compounds containing water molecules combined in a definite ratio as an integral part of the crystal. These hydrates are also said to contain water of crystallization or water of hydration. Each hydrate has a definite composition; a certain number of water molecules are combined with each formula unit of the salt. Examples of hydrates are:
| CuSO4•5H2O | Copper(II) sulfate pentahydrate |
| CoCl2 • 6H2O | Cobalt (II) chloride hexahydrate |
| ZnSO4 • 7H2O | Zinc sulfate heptahydrate |
Objective of the Experiment
This experiment is carried out to determine the percent (by mass) of water in a hydrate and the number of water molecules per formula unit of the hydrated salt.
Reagents and Apparatus
- Crucible
- Bunsen Burner
- Clay Triangle
- Retort Stand
- Tongs
- Weighing Balance
- Mesh
- Hydrate
Procedures
- Weigh a clean dry crucible (with its lid) to the nearest 0.001g. Add about 2g of the hydrate to the crucible and weigh the covered crucible to the nearest 0.001g.
- Place the crucible on the clay triangle with the lid ajar, so water vapor can escape. Heat very gently for few minutes, before increasing heat, and heat for 10 mins.
- Turn off burner and allow the crucible to cool to room temperature. Weigh the crucible to the nearest 0.001g.Reheat the crucible and contents for about 5 minutes and, after cooling, weigh it again. If the change in mass between the two weighing is more than 0.01g, repeat this heating, cooling and weighing sequence.
Results and Calculations
- Calculate the percentage of water using the formula below;
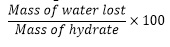
2. To determine the number of water molecules per formula unit of the hydrated salt, calculate the mole of water and the hydrate by dividing the mass by the molar mass. Calculate the mole ratio using the formula below;
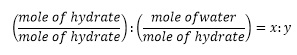
3. y (rounded to the nearest integer) is the number of water molecules per formula unit of the hydrated salt.
References
- Wikipedia (2018), “Hydrate”. Retrieved from http://en.m.wikipedia.org/wiki/Hydrate (accessed 11 June 2018).
- “Determine the Percentage of water in a hydrate”. Retrieved from https://salve.digication.com/alexanderantonopoulos?Lab_2_Determine_the_Percentage_of_Water_in_a_Hydrate (accessed 11 June 2018)
Download “Determination of Percentage of Water in a Hydrate”
DETERMINATION-OF-PERCENT-OF-WATER-IN-A-HYDRATE.docx – Downloaded 0 times – 21.90 KB