Introduction
When considering the suitability of water for town supply, the dissolved oxygen content is an important index. Good, clean potable water will give dissolved oxygen value close to the theoretical value for the saturated solution of oxygen in water. When there is pollution from organic matter and other trade effluents, the dissolved oxygen is up in various biochemical oxidation processes and its is only slowly replaced through surface absorption. Such water will give low dissolved oxygen content until oxidation is completed. Adequate dissolved oxygen is necessary for the life of fish and other aquatic organisms.
The methods described below for the determination of oxygen in water is based on that devised by Winkler. When manganese hydroxide is precipitated in the water sample it is quickly oxidized to higher hydrated oxides (probably in the four valent state) by the dissolve oxygen. Iodine, equivalent to the dissolved oxygen content, is then liberated on acidification in the presence of iodine, and it may be titrated with standard thio‐sulphate.
Objective(s) of the Experiment
To estimate the dissolved oxygen (DO) content in a water sample by Winkler’s method.
Reagents Used
- Manganese sulfate solution
Dissolve 100 g manganese sulfate (MnSO4 . 4H2O) in 200 cm3 distilled water. - Alkaline‐iodide‐azide solution
Dissolve 100g sodium hydroxide in 100 cm3 distilled water. Allow to stand for some days, during which any carbonate present sinks to the bottom. Siphon off all of the clear liquid, add 30g potassium iodide and 2g sodium azide and make up to 200 cm3 with distilled water. Store in plastic container. - Sodium Thiosulphate (0.0125 mol dm‐3)
Dilute 125 cm3 0.1 mol dm‐3 sodium thiosulphate to 1 liter with distilled water. - Potassium iodide solution ( 0.025 mol dm‐3 )
Dry analytical grade potassium iodate at 120oC. Dissolve 5.35g in distilled water and dilute to exactly 1 litre. This solution is stable for long periods if stored in a glass stopper bottle. - Potassium iodide solution (10% W/V)
Dissolve 10g KI in 100 cm3 distilled water. - Potassium fluoride solution (10% W/V)
Dissolve 10g potassium fluoride in distilled water and make up to 100 cm3 - Ammonium hydrogen carbonate solution
Dissolve 70g ammonium hydrogen carbonate in 185 cm3 distilled water. - Phosphoric acid (85% W/V )
Procedures
Interferences and Pre – Treatment
Most oxidising and reducing substances e.g dissolved organic substances, nitrite ions, higher‐valency manganese compounds, active chlorine, sulphide and sulphite ions, iron (II) and irons interfere. The influence of the dissolved organic substances can be excluded by conversion of the manganese hydroxides into oxygen‐sensitive carbonates by subsequent addition of 4cm3 ammonium hydrogen carbonate solution. Nitrite in acidic solutions catalyses the liberation of iodide and can be decomposed by addition of alkaline‐iodide‐azide solution. Iron (III) ions are rendered inactive during the determination by the addition of 4cm3 phosphoric acid or 2cm3 potassium fluoride solution.
Collection of Sample
Collect the sample in a narrow necked 200‐300 cm3 glass bottle having an accurately fitting ground glass stopper. If the water is from a tap, pass the water down a glass tube to the bottom of the bottle and allow water to overflow for 2‐3 minutes before insertion of the stopper. When sampling stream water, displace the water in the bottle several times, before collecting the sample. The water temperature, weather conditions and nature of the water sample at the time of sampling should be recorded. Avoid inclusion of air bubbles in the sample bottle.
Determination of the Dissolved Oxygen
- Carefully remove the stopper from the sample bottle
- Add in turn 1 cm3 manganese sulfate solution followed by 1 cm3 alkaline‐iodide‐azide solution. When introducing various reagents into the full bottle of sample, the tips of the pipettes should be well below the surface of the liquid.
- Replace the stopper carefully after each addition so as to avoid inclusion of air bubbles. If air bubbles are noticed, discard the sample and start over.
- If oxygen is present, a brown orange cloud of floc or precipitate will appear. When this floc has settled to the bottom, thoroughly mix the contents by inversion and rotation and let it settle again, until clear supernatant water is obtained.
- Add 1 cm3 concentrated sulphuric acid with the trip of the pipette below the level of solution and again replace the stopper.
- Mix well by rotation until the precipitate has completely dissolved.
- Pipette into a 250 cm3 conical flask 100 cm3 of the solution and immediately titrate it against standard sodium thiosulphate (0.0125 mol dm-3). Titrate by slowly dropping titrant solution from a calibrated pipette into the flask and continually swirling the sample water.
- When solution becomes pale yellow, add 2 or 3 drops of freshly prepared starch solution as the indicator so a blue color forms.
- Continue slowly titrating until the sample turns clear. As this experiment reaches the endpoint, it will only take one drop of the titrant to eliminate the blue colour. Be especially careful that each drop is fully mixed into the sample before adding the next. It is sometimes helpful to hold the flask up to a white sheet of paper to check for the absence of the blue color.
Results
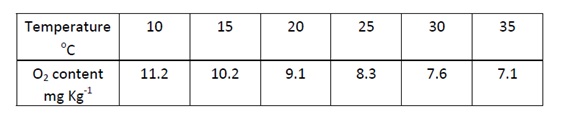
- Establish the relationship: 10 cm3 of 0.0125 mol dm‐3 sodium thiosulphate = 1 mg O2 (refer to the table below).
- Report the result in mg dm‐3 and as percentage saturation
References
- 1. Bruckner M.Z. (2018). “The Winkler Method – Measuring Dissolved Oxygen”. http://serc.carleton.edu/microbelife/research_methods/environ_sampling/oxygen.html Accessed March 3, 2018.
Download “Dissolved Oxygen in Water Determination”
Dissolved-Oxygen-in-Water-Determination.docx – Downloaded 0 times – 148.47 KB






